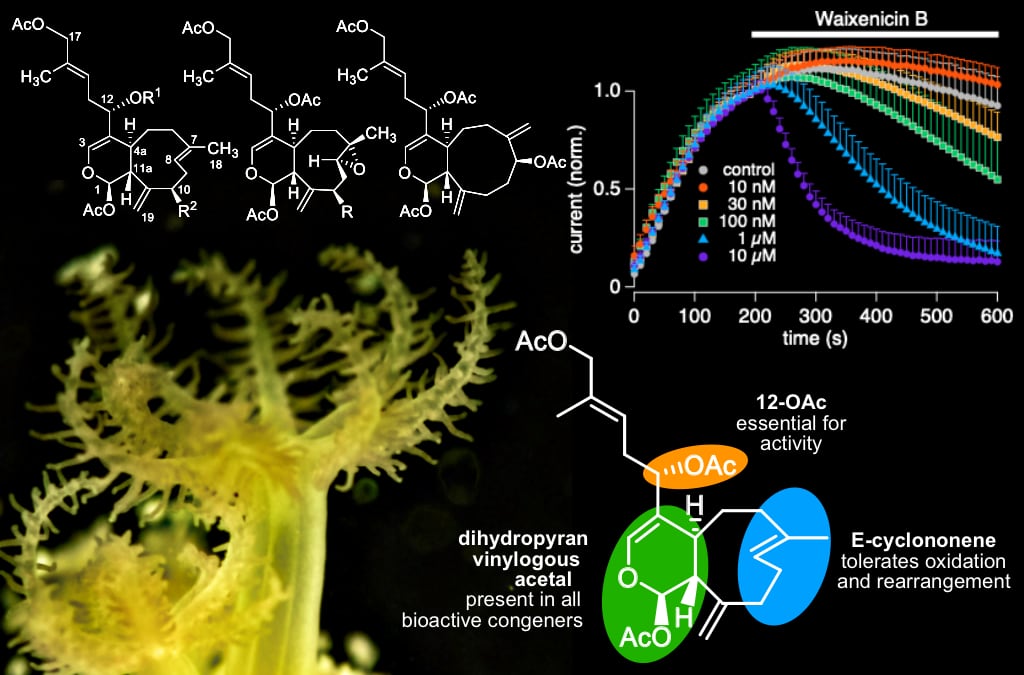
In this study, Drs. Suzuki and Fleig collaborated with a multi-disciplinary of chemists and physiologists to characterize the structure-activity relationships of waixenicin A, a xenicane diterpene from the Hawaiian coral Sarcothelia edmondsoni. Waixenicin A is a selective and potent inhibitor of the TRPM7 ion channel, which is emerging as a potential drug target for ischemic diseases and certain cancers. To study the structure-activity relationship (SAR) of waixenicin A, a multi-disciplinary team of natural product chemists and physiologists isolated and assayed related diterpenes from the coral. This preliminary SAR begins to define the essential structural features required for TRPM7 inhibition by the waixenicin A pharmacophore, and both the structural elements associated with activity and the in vitro bioassay data presented here support the hypothesis that waixenicin A inhibits TRPM7 activity through a covalent mechanism. These insights into the structural and conformational requirements for activity are informing the team’s ongoing medicinal chemistry efforts to develop waixenicin A derivatives as TRPM7 inhibitors with improved pharmacokinetics for subsequent drug lead development.