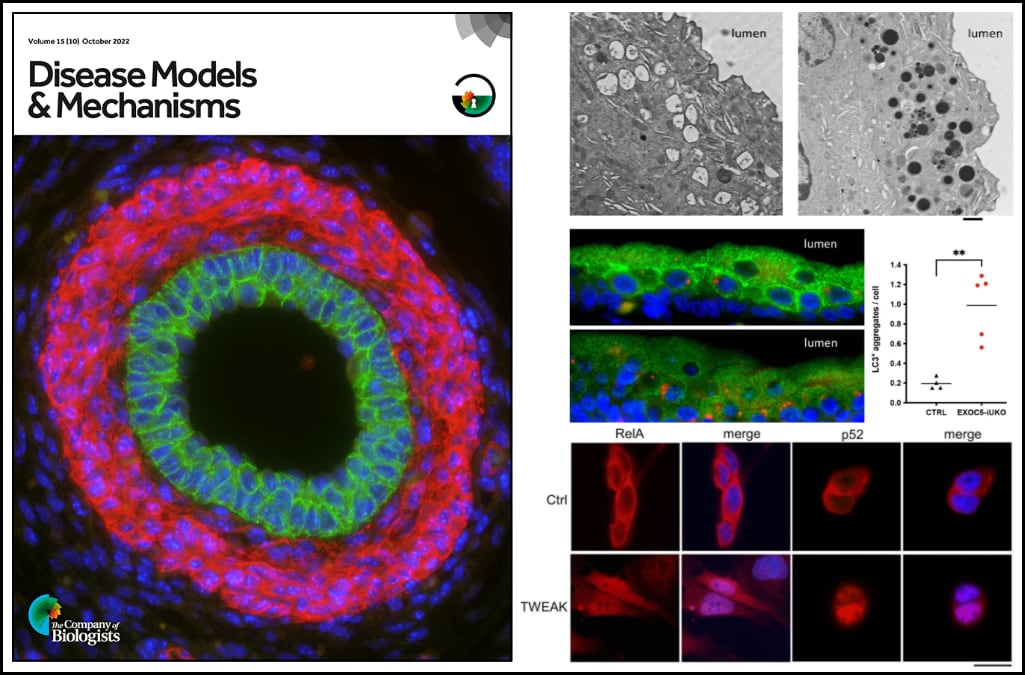
Dr. Ortega’s study investigates a unique Exoc5 conditional knockout mouse that develops obstruction of the urinary tract during embryo development. This blockage causes an accumulation of urine and an enlargement of the kidney (known as hydronephrosis) and is a common cause of chronic kidney disease. Currently, there is a limited understanding of what promotes for formation of ureter obstructions to during embryo development. This work features the first transgenic mouse model to display prenatal ureter obstruction and hydronephrosis.
Dr. Ortega and colleagues demonstrate that the exocyst plays a critical role in autophagy in urothelial cells, and that disruption of autophagy activates a urothelial NF-κB stress response. Impaired autophagy first provokes canonical NF-κB activity, which is progressively followed by increasing levels of non-canonical NF-κB activity and cell death if the stress remains unresolved. Thus, ablation of Exoc5 was found to disrupt autophagic stress response and thereby activate progressive NF-κB signaling, which promotes cell death and subsequently obstructive uropathy.
Furthermore, the authors found that cell death and ureter obstructions can be completely rescued by administering a single dose of the pan-caspase inhibitor z-VAD-FMK at embryonic day 16.5.
Data acquired for this study were featured on the cover of the journal: Immunohistochemical staining of an embryonic day (E)18.5 Exoc5 conditional knockout mouse ureter with E-cadherin (green), smooth muscle actin (red) and DAPI (blue) reveals complete rescue of in utero ureteropelvic junction obstruction with a single treatment of the broad-spectrum caspase inhibitor z-VAD-FMK at E16.5